Nitrogen
Nitrogen (N) has a pronounced and often dramatic influence on the growth and yield of crops. Management of soil and fertilizer N is difficult because N undergoes numerous transformations and is easily lost from the soil. These losses concern growers for three principal reasons: 1) N losses can and often do adversely affect plant growth and crop yield, 2) when N is lost in the nitrate form, there is a chance for contamination of groundwater and drinking water supplies, and 3) it is expensive to replace lost N.
The Nitrogen Cycle
This Nitrogen Cycle illustration shows nitrogen (N) inputs, losses and transformations. 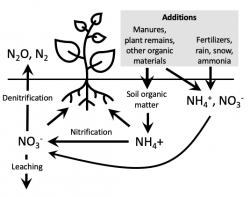
The Nitrogen Cycle
When inputs exceed plant needs, nitrates can accumulate in the soil and pose a threat to groundwater. Conversely, when plant-available forms of N from the soil and any inputs are too low, crop growth suffers. The key to successful management of N is to find the relatively “thin line” between too much and too little N. It is not an easy task. N transformations and losses are affected by soil conditions and the vagaries of the weather. The rates of most N inputs are difficult to accurately estimate.
Nitrogen Inputs
As can be seen from the N cycle, there are two sources of the N used by plants: ammonium (NH4) and nitrate (NO3). In addition to commercial fertilizer sources, available N may be added to the soil through mineralization (the microbial conversion of organic N to ammonium and then nitrate) of soil organic matter, manure and other organic residuals, and plant litter.
Soil organic matter: Organic matter contains the largest pool of soil N, usually comprising more than 90 percent of total soil N. The total amount of N in the plow layer of agricultural soils is surprisingly large. One can estimate the total N in pounds per acre in the 6″ to 7″ of surface soil by multiplying the soil’s organic matter content by 1,000. Thus, a soil with 4% organic matter contains about 4,000 lbs total N per acre.
The amount of this total N available to plants in any one year, however, is relatively small. Research has shown that for most soils 2% to 4% of the total N is converted (mineralized) annually to forms plants can use. For soil with a total of 4,000 lbs N per acre, a 2% – 4% conversion would produce 80 to 160 lbs N per acre annually for plant use. If the crop needs 200 lbs N per acre for adequate growth and development, some additional N must come from non-soil sources. Manure and/or fertilizer are the most likely candidates to furnish rapidly available N. The rate of mineralization is dependent on microbial activity, especially bacterial activity. Such activity is favored by warm soils with adequate, but not excessive moisture and a pH above 6.0. These conditions are also favorable to most fruit crops. On well-managed soils used for fruit production, 20 to 40 lbs of N per acre will become available during the growing season for each percent of organic matter if the weather is favorable.
Manures and other waste products: The N content of manures and their N fertilizer equivalents are highly variable. Differences in N content are due to the species of animal, the animal’s age and diet, the moisture content of the manure, handling and storage and the amount of bedding in the manure. The N fertilizer equivalent of a given manure varies not only with the animal species and the total N content of the manure, but also with the time of application (Table 3). The values in this table are based on numerous analyses of Connecticut manures as well as published data from other states. If specific manure analysis data for the farm are not available, growers should estimate N credits by the table. The time elapsed between spreading and incorporation is also important. About half of the N in dairy manure and three quarters of the N in poultry manure is in the form of ammonia, which is volatile. If left on the soil surface, this N will volatilize and be lost. To avoid this loss, manure should be incorporated shortly after spreading. NOTE: Manure often contains human pathogens. Serious illness has occurred from eating produce where fresh manure was applied without an adequate waiting period (see “Food Safety”).
Previous manure applications: Up to 50% of the total N in cow manure is available to crops in the year of application. Between 5% and 10% of the total applied is released the year after the manure is added. Smaller amounts are furnished in subsequent years. The quantity of N released the year after a single application of 20 tons per acre of cow manure is small (about 15 lbs N per acre). However, in cases where manure has been applied at high rates (30 to 40 tons per acre) for several years, the N furnished from previous manure increases substantially.
The buildup of a soil’s N-supplying capacity resulting from previous applications of cow manure has important consequences for efficient N management, two of which are:
- The amount of fertilizer N needed for the crop decreases annually;
- If all the crop’s N needs are being supplied by manure, the rate of manure needed decreases yearly.
With cage layer poultry manure, a higher percentage of the total N in the manure is converted to plant-available forms in the year of application. Consequently, there is relatively less carry-over of N to crops in succeeding years. This is due to the nature of the organic N compounds in poultry manure. This does not mean, however, that there is never any carry-over of N from poultry manure applications. If excessive rates of poultry manure (or commercial N fertilizers) are used, high levels of residual inorganic N, including nitrate, may be in the soil the following spring. High levels of soil nitrate in the fall, winter and spring have the potential to pollute groundwater and coastal sea water.
Previous crops: Cover crops can supply appreciable amounts of N to succeeding crops. Legumes, such as alfalfa and red clover, can provide 100 pounds or more of N to crops that follow. Other legumes, mixed grass-legume stands and grass sods supply less N to succeeding crops (Table 2). Keep in mind that most of the N is in the leaves, not the roots. If a legume hay crop is harvested, most of the N is removed from the field along with the hay.
| Previous Crop | Nitrogen Credit Lbs N per acre |
|---|---|
| Grass sod | 20 – 40 |
| “Fair” clover (20-60% stand) | 40 – 60 |
| “Good” clover (60-100% stand) | 60 – 90 |
| “Fair” alfalfa (20-60% stand) | 60 – 90 |
| “Good” alfalfa (60-100% stand) | 100 – 150 |
| Sweet corn stalks | 30 |
| “Good” hairy vetch winter cover crop | 120 – 150 |
Compost as a nutrient source: Finished compost is a dilute fertilizer, typically having an analysis of about 1-1-1 (N-P2O5-K2O). The nitrogen content of composts varies according to the source material and how it is composted. In general, nitrogen becomes less available as the compost matures. Nitrogen in the form of ammonium (NH4+) or nitrate (NO3-) is readily available, however in a finished compost there should be little ammonium, and any nitrate that is produced could have leached away, especially if the compost is cured or left out in the open. The majority of the nitrogen in finished compost (usually over 90%) has been incorporated into organic compounds that are resistant to decomposition. Rough estimates are that only 5% to 15% of the nitrogen in these organic compounds will become available in one growing season. The rest of the nitrogen will become available in subsequent years.
Synthetic fertilizers: Fertilizers used to supply N include urea (46-0-0), diammonium phosphate (DAP: 18-46-0), monoammonium phosphate (MAP: 11-48-0), urea-ammonium nitrate solution (UAN: 32-0-0), calcium ammonium nitrate, calcium nitrate, potassium nitrate and various manufactured and blended fertilizers such as 15-8-12, 15-15-15 and 10-10-10. In bulk blended or custom blended mixes, N-containing fertilizers with almost any grade can be provided.
| APPLICATION TIMING (lbs N/ton) | |||
|---|---|---|---|
| Kind of Manure | April/May1 | Fall Only2 | Other times3 |
| DAIRY (COW) | |||
| Solid | 5 | 2 | 3 |
| Liquid | 16 | 18 | 12 |
| POULTRY, CAGE LAYER | |||
| fresh (20-40% DM)4 |
16 | 5 | 8 |
| sticky-crumbly (41-60% DM) |
22 | 7 | 11 |
| crumbly-dry (61-85% DM) |
32 | 10 | 16 |
|
1 “April/May” credits refer to manure applied and incorporated in April and/or May for spring-planted crops and for manure applied and incorporated within four weeks of planting at times other than spring. 2 Use “fall only” values for manure applied in no-till or maintenance situations where the manure is not incorporated. 3 “Other times” means any time or any combination of times other than April/May or fall only for manure applied for spring-planted crops. 4 DM = Dry matter. |
|||
Nitrogen Losses
Nitrogen losses occur in several ways. The loss of available soil N not only costs growers money, it has the potential to negatively impact both air and water quality. Understanding the cause of N losses can help growers make management decisions to improve N use efficiency and minimize negative environmental impact.
Volatilization Losses: These losses occur mainly from surface-applied manures and urea. The losses can be substantial — more than 30% of the N in top-dressed urea can be volatilized if there is no rain or incorporation within two or three days of application. Losses are greatest on warm breezy days. Volatilization losses tend to be greater from sandy soils with pH values above 7.0. Incorporate manures right after applying them to avoid volatilization losses. Under the right conditions more than 50% of the ammonium N may be volatilized within the first 48 hrs. of applying manure if it is not incorporated.
Not only does volatilization reduce the fertilizer value of manure and urea, it can degrade air and water quality. Ammonia in the atmosphere can form particulates that contribute to smog. Ammonia emissions can also contribute to eutrophication of surface waters via atmospheric deposition.
Leaching Losses: Nitrogen can be lost by leaching in either the ammonium or nitrate form. Usually, much more N is leached as nitrate than as ammonium. Leaching losses are greatest on permeable, well-drained to excessively-drained soils underlain by sands or sands and gravel when water percolates through the soil. Percolation rates are generally highest when the soil surface is not frozen and evapotranspiration rates are low. Thus, October through early December, and late March and April are times when leaching potential is greatest. This is why nitrate remaining in the soil after the harvest of annual crops such as corn in September is particularly susceptible to leaching. The use of cover crops following cash crops can take up this residual N and prevent it from leaching. The N will then be released for crop use after the cover crop is plowed down in the spring. Of course, leaching can occur any time there is sufficient rainfall or irrigation to saturate the soil. This is why it is important to attempt to match fertilizer N application rates with crop N needs.
Denitrification Losses: These losses occur when nitrate is converted to gases such as nitrous oxide (N2O) and nitrogen (N2), when the soil becomes saturated with water. Poorly drained soils are particularly susceptible to such losses. In especially wet years on some soils, more than half the fertilizer N applied can be lost through denitrification. Favorable conditions for denitrification often occur in early spring and late fall. Minimizing the concentration of nitrate in the soil during these periods by delaying N application in the spring and planting cover crops in the fall will help reduce denitrification losses.
Immobilization: Immobilization occurs when soil micro-organisms absorb plant-available forms of N. The N is not really lost from the soil because it is held in the bodies of the microorganisms. Eventually, this N will be converted back to plant-available forms. In the meantime, however, plants are deprived of this N, and N shortages in the plants may develop. Immobilization takes place when highly carbonaceous materials such as straw, sawdust or wood chips are incorporated into the soil. Manure with large amounts of bedding may cause some immobilization.
Crop Removal of Nitrogen: In most cases, the greatest removal of N from the soil is via crop removal. Strawberries remove approximately 100 lbs of N per acre annually (in foliage and harvested fruit). On the other hand, mature highbush blueberries only remove approximately 50-60 lbs of N per acre (in foliage, wood and harvested fruit). Raspberries likely remove somewhere in between. Anticipated crop removal of N is one of the factors used in calculating N budgets and making N fertilizer recommendations. Depending on the crop, variable amounts of the N absorbed by the crop are returned to the soil after harvest in non-harvested plant parts. Strawberry renovation returns a significant amount of crop biomass plus straw mulch to the soil, contributing to the Nitrogen budget potential. Again cover crops can take up much of this N and hold it against leaching.
Phosphorus
Phosphorus (P) is referred to as P2O5 (phosphate) for the purposes of soil testing, fertilizer grades and recommendations. Among other important functions, phosphorus provides plants with a means of using the energy harnessed by photosynthesis to drive its metabolism. Deficiency can lead to impaired vegetative growth, weak root systems, poor fruit and seed quality, and low yield; however, excessive soil phosphorus levels are a concern due to the potential negative impact on surface water quality. Most phosphorus losses occur with runoff, but where soil levels are extremely high, subsurface losses can occur. Phosphorus enrichment is a leading source of water quality impairment of many lakes, streams, and rivers in New England.
Soil phosphorus exists in a wide range of forms. Some phosphorus is present as part of soil organic matter and becomes available to plants as the organic matter decomposes. Most inorganic soil phosphorus is bound tightly to the surface of soil minerals (e.g.., iron and aluminum oxides). Warm, moist, well-aerated soils at a pH level of about 6.5 optimize the release of both of these forms. Plants require fairly large quantities of phosphorus, but the levels of phosphorus available to plant roots at any given time are usually quite low. Soil tests attempt to assess the ability of soil to supply phosphorus from bound forms during the growing season. When a soil test indicates that phosphorus is low and fertilizer is needed, the rate recommended is intended to satisfy immediate crop needs and begin to build soil phosphorus levels to the optimum range (i.e., build and maintain). Phosphorus recommendations are customarily expressed as P2O5 to correlate with fertilizer analysis. Once soil test levels are in the optimum range, only a small amount of phosphorus is needed to replace the amount removed each year to maintain soil levels.
If your soil test results indicate above optimum levels, phosphorus application is unnecessary and should be limited. Where soil phosphorus levels are excessive, phosphorus application should be eliminated, and additional steps should be taken to minimize the risk of surface water contamination by limiting runoff losses.
Potassium
Potassium (K) is expressed as K2O similar to the way P is expressed as P2O5. Crop need for K varies. Plants use potassium to open and close stomates and to move nitrates from the roots to the leaves. Potassium rivals nitrogen as the nutrient absorbed in greatest amounts by plants. Like nitrogen, crops take up a relatively large proportion of plant-available potassium each growing season. Plants deficient in potassium are unable to utilize nitrogen and water efficiently and are more susceptible to disease. Most available potassium exists as an exchangeable cation (see below). The slow release of potassium from native soil minerals and from fixed forms in clays can replenish some of the potassium lost by crop removal and leaching. This ability, however, is limited and variable. Fertilization is often necessary to maintain optimum yields. See the table at the beginning of each crop section for the potassium needs for each crop.
It is important that the soil K plus the applied K is enough to meet crop needs. However, excessive levels should be avoided because K can interfere with the uptake of Ca and Mg (see “Base Saturation”). K is subject to leaching on sandy soils low in organic matter. If high amounts of K are needed, split applications should be used. Potassium sulfate (0-0-50) or sulfate of potash magnesium (Sul-Po-Mag, 0-0-22) are the best sources of potassium for brambles and strawberries. Although muriate of potash (KCl, 0-0-60) is less expensive, brambles are sensitive to the chloride in this fertilizer.
Calcium
Calcium is usually supplied in sufficient quantities by liming if appropriate liming materials are chosen (see “Soil pH and Exchangeable Acidity”). If soil pH is high and Ca is needed, small amounts can be applied as calcium nitrate fertilizer (15% N, 19% Ca). Ca can also be applied without affecting pH by applying calcium sulfate (gypsum, 22% Ca) or superphosphate (14 to 20% Ca).
Magnesium
Magnesium is necessary for chlorophyll production and nitrogen metabolism. High soil potassium levels can lead to reduced uptake of magnesium. Magnesium deficiency is characterized by interveinal reddening on older leaves, beginning at the leaf margin. It is important to maintain a proper balance between magnesium, potassium, and calcium. These three nutrients and phosphorus can be applied in late fall after plants are dormant. Nutrients can then move into the root zone and be available when growth begins again in the spring. Magnesium (Mg) is most economically applied as dolomitic or high-mag limestone (see “Soil pH and Exchangeable Acidity”). If liming is not needed, Sul-Po-Mag (11% Mg, 22% K) can be used. You can order blended fertilizer containing Mg.
Minor Elements
Minor elements are difficult to analyze accurately with soil tests. Plant tissue analyses are more reliable for determining whether or not plants are getting sufficient quantities of minor elements. Of the minor elements, boron (B) and Zinc (Zn) are the most likely to be needed to supplement soil levels.
Special Note on Lead
Many laboratories routinely screen all soil samples for elevated levels of extractable lead. Lead is naturally present in most New England soils at low concentrations (15-40 ppm total lead). At these levels lead generally is thought to present minimal danger to people or plants. Soil pollution with lead-based paint and the tetraethyl lead of past automotive fuels have increased soil lead levels to several thousand ppm in some places. Unless the estimated total lead level in your soil exceeds 299 ppm (Modified Morgan extractable level of 22 ppm) it is simply reported as low and can be considered safe (assuming the sample submitted is representative of the area of concern). Estimated total lead levels above 300 ppm are a concern. In such cases, consult your state’s Extension Service for further assistance or see: “Soil Lead: Testing, Interpretation, & Recommendations” for more information on soil lead testing and recommendations.
| CROP | AGE | AMOUNT/TIMINGS (ACTUAL N) | N SOURCE | COMMENTS |
|---|---|---|---|---|
| Strawberries | 0 | 30 lb/A early June 30 lb/A early Sept. |
ammonium or calcium nitrate | Be sure plants are growing well prior to application |
| 1+ | 70 lb/A at renovation 30 lb/A early Sept. |
ammonium or calcium nitrate or urea | Adjust fall amount based on leaf tissue analysis | |
| Raspberries and Blackberries (summer bearing) |
0 | 25-35 lb/A 4 weeks after planting | calcium nitrate | Avoid touching plants with fertilizer |
| 1 | 35-55 lb/A split between May and June | ammonium nitrate or urea | Use higher amount on sandier soils or if irrigation is used | |
| 2+ | 40-80 lb/A split between May and June | |||
| Raspberries (fall bearing) |
0 | 25 lb/A 4 weeks after planting | calcium nitrate | Avoid touching plants with fertilizer |
| 1 | 50-80 lb/A split between May and June | ammonium nitrate or urea |
Use higher amount on sandier soils or if irrigation is used. Adjust based on leaf tissue test on mature plantings |
|
| 2+ | 70-100 lb/A split between May and June | |||
| Blueberries | 0 | Do not fertilize newly planted blueberries |
Soil pH should be adjusted to 4.5-5.0 prior to planting Use ammonium sulfate where soil pH is >5.0 Adjust based on leaf tissue test on mature plantings |
|
| 1 | 15 lb/A, split between May and June | ammonium sulfate or urea DO NOT use aluminum sulfate |
||
| 2 | 20 lb/A, split between May and June | |||
| 3 | 25 lb/A, split between May and June | |||
| 4 | 35 lb/A, split between May and June | |||
| 5 | 45 lb/A, split between May and June | |||
| 6 | 55 lb/A, split between May and June | |||
| 7+ | 65 lb/A, split between May and June | |||
| Currants and Gooseberries | 0 | 25 lb/A, 4 weeks after planting | calcium nitrate | |
| 1 | 50-80 lb/A, split between May, June, August | calcium nitrate | ||
| 2+ | 70-100 lb/A, split between May and early August | calcium nitrate | ||
| Elderberries | 0 | Do not fertilize newly planted elderberries | ||
| 1+ |
Apply 1/8 lb of ammonium nitrate for each year of the plant’s age, up to one pound per plant |
ammonium nitrate or 10-10-10 |
In spring, spread fertilizer with a spreader in bands one foot wide along both sides of the rows. | |
| Juneberries | 0 | 25 lb/A, 4 weeks after planting | calcium nitrate | Avoid touching plant with fertilizer after planting. |
| 1 | 50-80 lb/A, split between May and June | urea or ammonium nitrate | Use higher amount of sandier soils or if irrigation is used. | |
| 2+ | 70-100 lb/A, split between May and June | urea or ammonium nitrate | Use higher amount on sandier soils or if irrigation is used. Adjust with leaf tissue analysis. |
|
| Source: 2016 Cornell Pest Management Guidelines for Berry Crops | ||||
